Inner-shell spectroscopy
Inner-shell spectroscopy provides a singular way to study structure of molecules. While visible and ultraviolet spectra strongly depend on the overall structure of the molecular system, the orbitals involved in the inner-shell excitation processes are very localized and strongly resemble the orbitals of isolated atoms. This leads to relatively simple spectra, which have well defined regions concerning to each different element present in the system.
If a molecule has two or more atoms of the same element in nonequivalent positions, changes in the energy spectrum and other molecular properties are expected and, in principle, they may be mapped so as to establish a relation connecting the different molecular environment to the measured property.
In collaboration with experimentalists from the McMaster University and researchers from the Federal University of Rio de Janeiro, we investigated the inner-shell excitation induced by electron impact of the molecules listed bellow.
|
Molecule |
Excited shells |
Ref. |
|
CH≡CH (acetylene) |
C 1s |
|
|
CH2=CH2 (ethylene) |
C 1s |
|
|
N2O (nitrous oxide) |
N 1s, O 1s |
|
|
CO2 (carbon dioxide) |
O 1s |
|
|
C4H6 (butadiene) |
C 1s |
|
|
CS2 (carbon disulfide) |
S 2p, C 1s |
The main quantity used to characterize the inner-shell excitations is the generalized oscillator strength (GOS) which is given as a function of the transferred momentum K by
![]() , (1)
, (1)
where E is the transition energy, gn is the degeneracy of the final state, and R0 is the equilibrium geometry, The integration over Ω results from the averaging of the orientation of the molecule with respect to K. The transition amplitude ε0n is given by
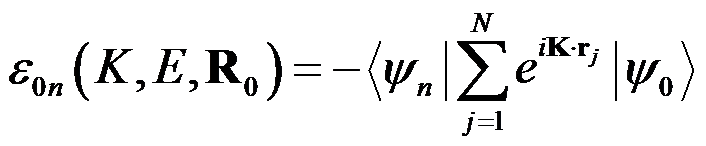 , (2)
, (2)
where rj are the coordinates of each of the N molecular electrons. ψ0 and ψn are the electronic wavefunctions of the ground and final excited states.
|
|
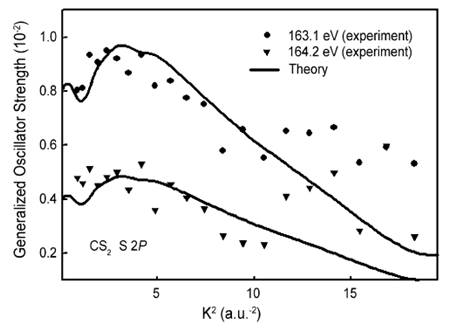
Experimental and theoretical GOS for the S 2p → 3πu* excitation of CS2.[27] |
As illustrated in the figure above, the theoretical results can reproduce the main features of the experiments, which make them a valuable tool for the interpretation of this kind of spectrocopy.
The calculation of the electronic wavefunctions of inner-shell-excited molecules imposes a great challenge and demands quite complex ab initio methodologies.
Young-type interference in inner-shell excitations
Often, experimentalists have observed oscillations in the GOS profiles as a function of transferred momentum. We have shown by means of a simple analytical model that these oscillations are analogous to the interference patterns in the Young's double-slit experiment. According to this model, the GOS for a symmetric molecule is simply given by [15]
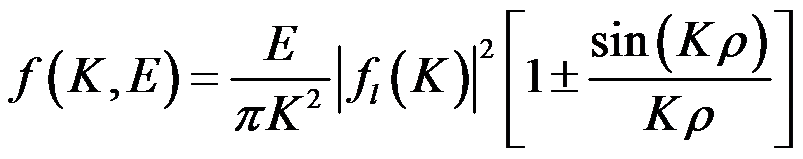 , (3)
, (3)
where fl is the scattering atomic amplitude and ρ is the distance between the symmetric atoms.
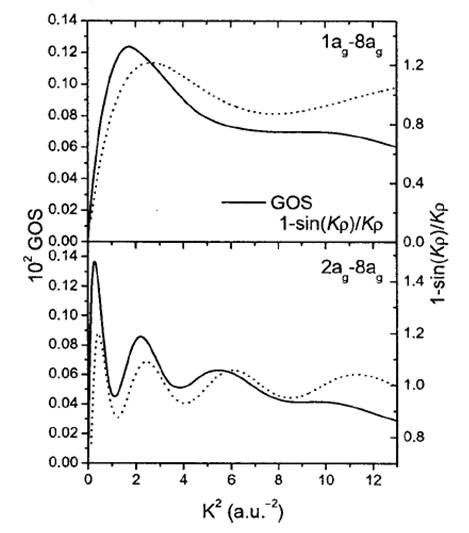
The figure bellow shows that the GOS oscillations have the same origin as Young oscillations. This implies that the scattered electron behaves as a wave during its interaction with the molecule, as it does when it is is propagated through a double slit.
 |
|
GOS computed with Eq. (1) (solid line) compared to the oscillatory function of the Young interference given in Eq. (3) (dotted line) for butadiene.[15] |