Nonadiabatic phenomena
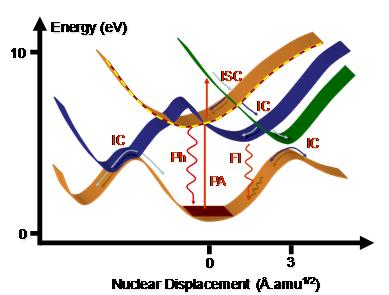
As a consequence of a photoabsorption (PA) in the UV or visible region of the spectrum, a molecule is activated to an excited electronic state. Immediately after the excitation, it starts to relax by following many different deactivation mechanisms.
The molecule can undergo an internal conversion (IC) to other states of same spin multiplicity or make an intersystem crossing (ISC) to a state of different multiplicity. From the excited state minima it can return to the ground state either by emission of a photon through fluorescent (Fl) and phosphorescent (Ph) processes, or it can deactivate without emitting radiation via internal conversion. The crossing between states where the internal conversion occurs is called a conical intersection.
|
|
|
Schematic illustration of the deactivation mechanisms in a UV/vis excited molecules. |
Each of these processes occurs in different time scales. The fastest, the internal conversion, may occurr within few picoseconds (10-12 s) and usually involves conformers for which the Born-Oppenheimer approximation breaks down. The slowest, phosphorescence, may take microseconds (10-6 s). The preferential processes dominating each molecule depend upon the specific shapes of the excited state potential energy surfaces.
Based on our dynamics simulations results, we have reviewed in Ref.[33] how the internal conversion takes place in small organic molecules
Ethylene and substituted ethylenes
The internal conversion in many organic molecules occurs by excitation of a ππ* state in a set of double bonds. For this reason it is very important to understand how the internal conversion takes place in the simplest models for a double bond, ethylene and substituted ethylenes.
We have performed quantum chemical and excited state dynamics investigation of few of these small molecules, including ethylene (CH2=CH2) itself,[11,14,16] fluoroethylene (CH2=CHF),[13] silaethylene (CH2=SiH2), [21] and methaniminium cation (CH2=NH2+).[18,31]
These investigations have revealed that differently from what is generally believed, the deactivation is usually not done through a single pathway, but though many deactivation pathways driving the molecule to different conical intersections.
Heterocycles
Any molecule has conical intersections connecting the excited and the ground state. Whether these intersections will be used for internal conversion or the molecule will deactivated by emission of a photon depends only on how are the shapes of the surfaces of potential energy in the excited states.
Pyridone is an example of a fluorescent species which does not access its conical intersection. Following the experimental results of Leutwyler and co-workers, our simulations have shown that if a bias towards the out of plane vibrational modes is added right in the beginning of the dynamics, the conical intersections can be reached and the internal conversion rate increases.[30]
Pyrrole [19,45,48,57] and imidazole [41] are non-fluorescent five-membered heterocycles. Using excited-state dynamics simulations, we have shown the statistical importance of each of the several deactivation pathways available for these molecules.
An important class of heterocycles undergoing internal conversion is the DNA/RNA nucleobases. Their ultrafast deactivation may represent a natural protection of the genetic code against UV excitation. The dynamics of nucleobases and base models is discussed in "Internal conversion of DNA/RNA nucleobases" page.
Formamide
Formamide is the simplest model for a peptide bond, which binds together the amino acids in a protein. For this reason it is interesting to understand how it reacts to the UV excitation. Headed by our collaborators in Zagreb (M. Eckert-Maksic, I. Antol, and M. Vazdar), we have investigated the dynamics of formamide,[22,28] protonated formamide,[29] and of formamide in an Ar Matrix.[56] The multiple reaction pathways and their statistical importance have been mapped in each case.
In a recent collaboration with the experimental group of W. Sander,[81] we have shown that the major product of the dissociation dynamics of methyl-formamide in Ar matrix is a weakly-bound complex between CH3NH and HCO radicals. This complex owes its existence to the cage effect of the matrix which allows for H-transfer reactions and recombination
|
|
Excited-state dynamics simulations of formamide in Ar matrix. The dynamics starts in the S1 state and runs for 2 ps. Complex formation followed by hydrogen transfer is observed.[56] |
Protonated Schiff bases
It has been determined in the 1960's that the initial molecular steps are the absorption of light by retinal, the chromophore of the rhodopsin, a protein present in the retina, which isomerizes as a consequence of the excitation.
Since then, the detail of the isomerization cycle of rhodopsin and other similar photoreceptors have became one of the most active fields of research in photochemistry and photobiology. The simplest models for retinal are the protonated Schiff bases known as PSBn. We have investigated the nonadiabtaic dynamics of PSB3 [36] and PSB4 [46] by ab initio methods, uder a variety of environment restrictions. One of our main findings is the "Folding Table" isomerization mechanism, which takes place in PSB3 and PSB4 under large degree of restriction. This mechanism is shown in the movie below.
Folding Table isomerization mechanism occurring in 200 fs of dynamics of PSB4.[46] |
Azobenzene & Azomethane
Azobenzene (Ph-N=N-Ph) and azomethane (CH3-N=N-CH3) are prototypes of molecular phototriggers.
We have investigated the nonadiabatic cis-to-trans isomerization of azobenzene after S1 (n–π*) excitation, employing the fewest-switches surface hopping method based on ab initio methods.[69] Azobenzene photoisomerization occurs purely as a rotational motion of the central CNNC moiety. Two nonequivalent rotational pathways corresponding to clockwise or counterclockwise rotation are available. The course of the rotational motion is strongly dependent on the initial conditions. The internal conversion occurs via an S0/S1 crossing seam located near the midpoint of both of these rotational pathways. Based on statistical analysis, we showed that the occurrence of one or other pathway can be completely controlled by selecting adequate initial conditions.
The excited-state dynamics of azomethane is a ultrafast process leading to a clean formation of CH3 radicals. We have investigated the details of the initial steps in this process. First, we characterized the UV spectrum of this molecule, [61] and then the first picosecond of dynamics up to the internal conversion in gas phase[53] and in solution.[60]