Dynamics of nucleobases
The five nucleobases occurring naturally in DNA and RNA (ade, gua, cyt, thy, ura) strongly absorb UV radiation. They all are non-fluorescent species which return to the ground state by internal conversion within few picoseconds (1 ps = 10-12 s). It is possible that the short lifetime of the nucleobases excited-states is constitutes natural protection of the genetic code against UV induced mutations.
We have investigated how the internal conversion takes place in which of the five nucleobases.[32,37,47,59,64,65,69] These investigations have been carried out with ab initio non-adiabatic dynamics simulations.
The movie below illustrates the internal conversion process for adenine.[32] After the excitation adenine quickly relaxes in the ππ* excited state. After few hundreds of femtoseconds (1 fs = 10-15 s) the pyrimidine ring undergoes a strong out of plane deformation at the carbon C2. This deformation promotes the conical intersection between the excited and the ground state where adenine deactivates.
|
|
First 200 fs of dynamics of adenine after UV excitation.[32] The graph on the top shows the potential energy as a function of time. The black dot indicates the current state every time step. The two graphs below indicate the evolution of the Cremer-Pople dynamics during the dynamics. This is one trajectory example. All other simulated trajectories (60 in total) behave pretty much in the same way. |
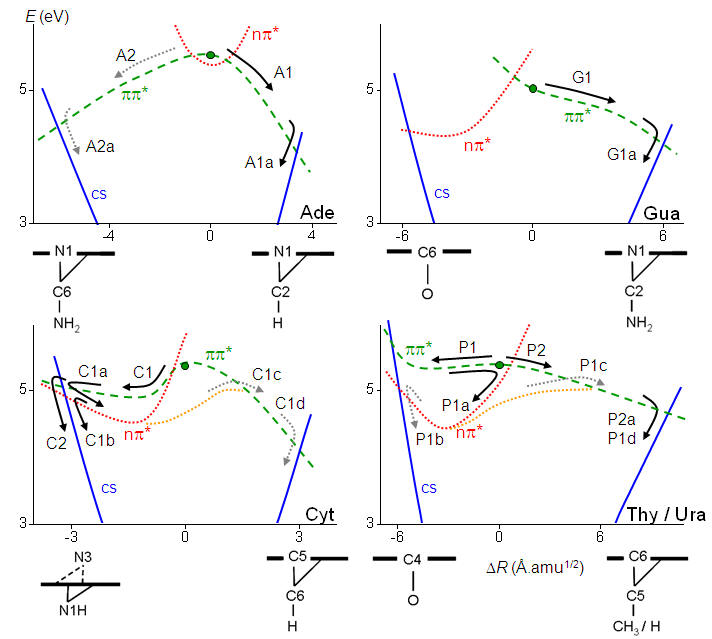
In spite of similar deactivation times, each of the five nucleobases follows different pathways. The purine bases adenine and guanine follow homogeneous pathways based on relaxation along the ππ* states. The pyrimidine bases (thymine, cytosine and uracil) follow inhomogeneous pathways, with sequences of relaxations into local excited-state minima and activation of multiple reaction pathways. These pathways are illustrated in the figure below, which are discussed in details in Ref.[59]
.|
|
Schematic view of the internal conversion pathways for the five natural nucleobases.[60] |
Quality of the simulations
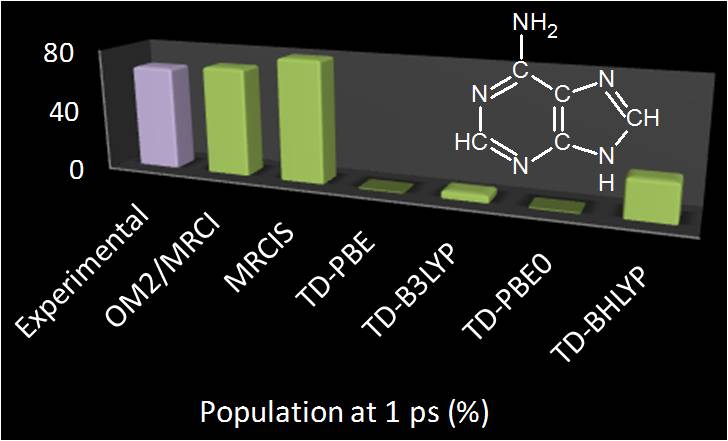
In spite of the importance of nonadiabatic dynamics simulations for the understanding of ultrafast photo-induced phenomena, simulations based on different methodologies have often led to contradictory results. In Ref.[79], we proceed through a comprehensive investigation of on-the-fly surface-hopping simulations of adenine in the gas phase using different electronic-structure theories (ab initio, semiempirical, and density functional methods, see figure below). Simulations that employ ab initio and semiempirical multireference configuration interaction methods predict the experimentally observed ultrafast deactivation of adenine with similar time scales, however, through different internal conversion channels. Simulations based on time-dependent density functional theory with six different hybrid and range-corrected functionals fail to predict the ultrafast deactivation. The origin of these differences is analyzed by systematic calculations of the relevant reaction pathways, which show that these discrepancies can always be traced back to topographical features of the underlying potential energy surfaces.
|
|
Experimental and theoretical predictions of the ground state population of adenine 1 ps after the photoexcitation.[79] |
Base models
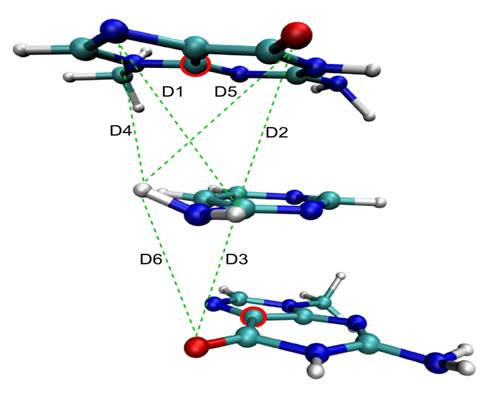
To understand the behavior of the nucleobases after UV excitation it has been useful to simulate not only the nucleobases themselves, but also nucleo base models. Molecules like aminopyrimidine and di-aminopyrimidine have been important to allows us to determine the role of the several different reaction pathways and conical intersection available for each nucleobase.[24,38,50,55,58] For instance, we have recently shown that the stacking interaction typical in nucleic acid strands does not inhibit the out plane motion which brings the nucleobases to conical intersections. These conclusion where achieved with QM/MM simulations of aminopyrimidine locked between two guanine bases, as shown in the figure below.[55]
|
|
Dynamics simulations of aminopyrimidine stacked between two guanines. [55] |
Survey of investigations
|
Molecule |
Refs. |
|
Adenine |
|
|
Guanine |
|
|
Cytosine |
|
|
Thymine |
|
|
Uracil |
|
|
Aminopyrimidine |
|
|
Pyridone |
|
|
Imidazole |
|
|
Diaminopyrimidine |
|
|
Diaminopurine |