Hydrogen ionic clusters X+(H2)n
The presence of a cation in a molecular hydrogen environment leads to the formation of molecular clusters around the ion. In the case of an H2 homogeneous atmosphere, an H2+ ion is quickly converted to the H3+ molecule, which becomes the core for the clustering process
H3+ + (n + 1)H2 → H3+(H2)n + H2 ,
where the exceeding H2 molecule carries away the excess of energy, stabilizing the cluster. From these multiple-step reactions, hydrogen clusters are formed, and clusters as large as n = 48 have been experimentally observed. Depending on the temperature and pressure conditions, clusters with n = 6 and 7 are the more abundant.
Recently, Barbatti and Nascimento have shown that the smallest cluster in the series, H3+(H2) or H5+, should play an important role in the chemistry of dense interstellar clouds.[75] Using a chemical-kinetical model based on computational and experimental results, they concluded that the concentration of H5+ in those clouds should be at least 10% of the concentration of H3+.
 |
|
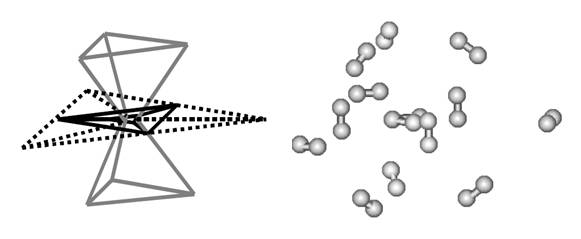
Equilibrium geometry of the H3+(H2)12 cluster as determined by ab initio calculations.[4] The H2 molecules are distributed in three distinct shells as the scheme at the left side shows. |
One of the most interesting features of these species is that in the cluster, each H2 molecule is strongly bound by the Coulombic field of the cation, which make the hydrogen ionic clusters potentially useful for dealing with hydrogen storage problems.
During his PhD research at the Federal University of Rio de Janeiro, Mario Barbatti investigated the structural properties of hydrogen clusters formed around diverse cations and with different sizes. A survey of these investigations is given in the Table below.
|
Cluster core X+ |
Cluster size n |
Properties |
Ref. |
|
H3+ |
1 - 3 |
Infrared spectra |
|
|
H3+ |
5 - 7 |
Structure, thermochemistry |
|
|
H3+ |
8 - 12 |
Structure, thermochemistry |
|
|
H3+ |
1 - 14 |
Fragmentation |
|
|
H3+ |
1 |
Interstellar chemistry |
|
|
Li+ |
1 - 7 |
Structure, thermochemistry |
|
|
Na+ |
1 - 7 |
Structure, thermochemistry |
|
|
K+ |
1 - 3 |
Structure, thermochemistry |
|
|
Li3+ |
1 - 6 |
Structure, thermochemistry |
|
|
Several |
Review |
Energy loss and crystal channeling
When a beam of charged particles traverses a crystal, its interactions inside the material can depend strongly on the target orientation. In particular, when the incidence direction is parallel to some of the crystal planes and axes, the incident particles are guided by the repulsive potentials associated to these structures, reducing the number of frontal collisions with the atoms of the crystal. This constitutes the well-known phenomenon of planar and axial channeling, which is reflected in a large reduction of the rate of energy loss of the particle inside of the material in comparison the the rate when the particle reaches the material in a non-channeling orientation.
We have built a semi-classical model to simulate the angular dependence of the electronic energy loss of fast ions between a channeling and a non-channeling direction. These models are compared to the measured energy loss of 2.0 MeV He ions channeled through a thin silicon crystal into directions that scan the {001} plane from the [110] axis to the [100] axis.[2]
 |
|
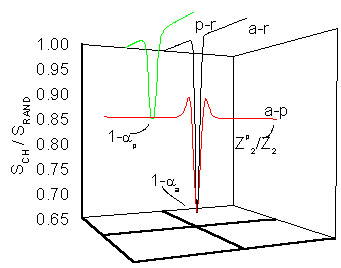
Simulated angular dependence of the energy loss of a 2 MeV He+ ion crossing a Si crystal in three different channeling directions. Values relative to the energy loss along a non-channeling direction.[2] |
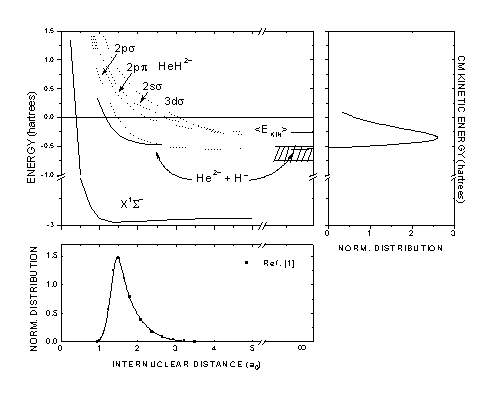
Collisional fragmentation of HeH+
We studied the cross section for H- ion production resulting from the collision reaction
HeH+ + X → He2+ + H- + X,
where X are He, Ne or Ar target atoms with velocities of 2.8, 4.0 and 4.9 a.u. From the H- kinetic energy distribution in the projectile center-of-mass frame, the potential energy curve associated to this dissociative channel was estimated.[1]
The analysis of the results shows that internal conversion processes between the present channel and several states of the HeH2+ ion leads to an inhibition of H- formation.
 |
|
Based on the internuclear distances distribution (bottom) and on kinetic energy distribution (right), the potential energy surface for the H- channel was built.[1] |